Dosing DOJOLVI (triheptanoin).
UNDERSTANDING DOJOLVI DOSING
Dosing for DOJOLVI is individualized based on patient tolerability, medical needs, and other dietary requirements.1,2
Download Additional Details

HOW THE DOJOLVI DOSE IS CALCULATED2
The recommended target daily dosage of DOJOLVI (triheptanoin) is up to 35% of the patient’s total prescribed daily caloric intake (DCI), divided into four or more doses and mixed thoroughly into semisolid food/liquid or medical food/formula at mealtimes or with snacks.*
Assess the metabolic requirements of the patient by determining their DCI prior to calculating the dose of DOJOLVI.
To find the DOJOLVI dose for your patient:
1
2
3
4
1. MULTIPLY
MULTIPLY the total DCI (in kcal) by the target percentage of the DCI that will be provided by DOJOLVI

2. DIVIDE
DIVIDE by 8.3 kcal/mL, the caloric value of DOJOLVI
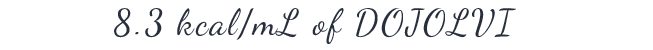
3. ROUND
ROUND the calculated total daily dosage of DOJOLVI (in mL) to the nearest whole number

4. DIVIDE
DIVIDE the total daily dosage into at least 4 approximately equal doses to be given at each interval mixed thoroughly with a meal or snack

In order to reach a target daily dosage, patients may require an increase in their total fat intake. All patients treated with DOJOLVI should be under the care of a clinical specialist knowledgeable in appropriate disease-related dietary management based upon current nutritional recommendations.
The neonatal population may require higher fat intake and therefore an increased amount of DOJOLVI. Current nutritional recommendations should be considered when dosing the neonatal population.
Initiating and titrating DOJOLVI2
For patients not currently taking a medium-chain triglyceride (MCT) product:
Initiate DOJOLVI (triheptanoin) at a total daily dosage of approximately 10% DCI, divided into at least 4 times per day. Increase to the recommended total daily dosage by approximately 5% DCI every 2 to 3 days until the target dosage of up to 35% DCI is achieved.
For patients switching from another MCT product:
Discontinue use of MCT products before starting DOJOLVI.
Initiate DOJOLVI at the last tolerated daily dosage of MCT divided into at least 4 times per day. Increase the total daily dosage by approximately 5% DCI every 2 to 3 days until the target dosage of up to 35% DCI is achieved.
Tolerability:
Consider more frequent, smaller doses if a patient has difficulty tolerating 1/4 of the total daily dosage at one time based on gastrointestinal adverse reactions.
Monitor the patient’s total caloric intake during dosage titration, especially in a patient with gastrointestinal adverse reactions, and adjust all components of the diet as needed.
If a patient experiences gastrointestinal adverse reaction(s), consider dosage reduction until the gastrointestinal symptoms resolve.
If a patient is unable to achieve the target daily dosage of up to 35% DCI during dosage titration, maintain the patient at the maximum tolerated dosage.
Do not administer DOJOLVI alone to avoid gastrointestinal upset and feeding tube degradation.
USING THE DOJOLVI CALCULATOR
Designed to help determine the DOJOLVI daily dosing schedule for your patients, the DOJOLVI Dosing Calculator can be used to:
- Determine dosing for all patients ready to start DOJOLVI. This includes patients:
- Not currently taking another medium-chain triglyceride (MCT) product
OR - Switching from another MCT product
- Not currently taking another medium-chain triglyceride (MCT) product
- Output reports for your records
- Support your patients with a customized dosing schedule
Calculate DOJOLVI Now
Administering DOJOLVI2
Administer DOJOLVI (triheptanoin) at least 4 times per day orally or via oral or enteral feeding tubes manufactured of silicone or polyurethane
Use an oral syringe or measuring cup made of compatible materials to withdraw the prescribed volume of DOJOLVI from the bottle
- Compatible materials include stainless steel, glass, high-density polyethylene (HDPE), polypropylene, low-density polyethylene (LDPE), polyurethane, and silicone
- Do not prepare or administer DOJOLVI using containers, oral syringes, or measuring cups made of polystyrene or polyvinyl chloride (PVC) plastics
- Regularly monitor the containers, dosing components, or utensils that are in contact with DOJOLVI to ensure proper functioning and integrity
Add the prescribed amount of DOJOLVI to a clean bowl, cup, or container made of compatible materials, which contains an appropriate amount of semisolid food/liquid or medical food/formula, and mix or emulsify thoroughly
To minimize gastrointestinal upset when taken orally, always mix DOJOLVI thoroughly with semisolid food or liquid such as:
- Plain or artificially sweetened fat-free yogurt
- Fat-free milk, formula, or cottage cheese
- Whole-grain hot cereal
- Fat-free, low-carbohydrate pudding, smoothies, applesauce, or baby food
The unused DOJOLVI mixture may be stored for up to 24 hours in the refrigerator.
If not used within 24 hours, dispose of the DOJOLVI mixture in the trash. Do not pour down the sink. Do not save for later.
If a dose is missed, take the next dose as soon as possible, with subsequent doses taken at 3- to 4-hour intervals. Skip the missed dose if it will not be possible to take all 4 doses in a day.
To help avoid gastrointestinal upset and feeding tube degradation, mix or emulsify DOJOLVI thoroughly into the medical food or formula. Do not administer DOJOLVI alone.
DOJOLVI is administered as an oral or enteral bolus medication. Do not add DOJOLVI to the feeding bag, as the feeding equipment may degrade over time. Feeding tube performance and functionality can degrade over time depending on usage and environmental conditions. In clinical trials, feeding tube dysfunction was reported in patients receiving triheptanoin.
Avoid administration of DOJOLVI in patients with pancreatic insufficiency.
For complete information on the preparation and administration of DOJOLVI, please refer to Section 2.4 of the full Prescribing Information.
PREPARING DOJOLVI
A video guide for your patients to demonstrate how to use the dosing components to prepare a DOJOLVI dose
Inserting the press-in bottle adapter
MIXING DOJOLVI
A video demonstration for your patients on how to properly mix DOJOLVI into semi-solid foods or liquids when preparing to take the medication orally
Do not administer DOJOLVI alone to avoid gastrointestinal upset. If the DOJOLVI mixture is not used within 24 hours, throw away (dispose of) in the trash. Do not pour down the sink. Do not save for later.
For complete information on the preparation and administration of DOJOLVI orally or enterally via a silicone or polyurethane feeding tube, please refer to Section 2.4 of the full Prescribing Information.
Inserting the press-in bottle adapter
Storing DOJOLVI2
DOJOLVI is a clear, colorless to light yellow liquid supplied in a 500 mL bottle containing 100% w/w of triheptanoin.
Store DOJOLVI upright at room temperature between 68° and 77°F (20° and 25°C)
Do not freeze DOJOLVI
Opened bottles of DOJOLVI can be used for up to 9 months after opening, but not beyond the expiration date on the bottle
Do not store DOJOLVI in containers made of polystyrene or polyvinyl chloride (PVC)

dosing guide
Provide your patients and their caregivers with a step-by-step guide for storing, administering, and keeping track of DOJOLVI doses.

Download the Guide
References:
- Data on file. Ultragenyx CL201 CSR. Ultragenyx Pharmaceutical Inc.; 2017.
- DOJOLVI (triheptanoin) US Prescribing Information; October 2023.