Support for you and your patients in one location.
PATIENT SERVICES FOR DOJOLVI
Have confidence in availability of DOJOLVI and access for your LC-FAOD patients
More than 400 patients in the US with LC-FAOD have been prescribed DOJOLVI (since FDA approval in June 2020)1
Ultragenyx is committed to patients with rare diseases, which is why we created UltraCare Patient Services for DOJOLVI—your patients’ guide throughout their treatment journeys.
Our UltraCare Guides have varied backgrounds, including social workers, healthcare professionals, and patient advocates, and are ready to help patients gain and maintain access to DOJOLVI.
UltraCare Guides help patients and their caregivers:
Understand their
insurance coverage
Utilize patient support program resources, including help with billing and coding
Find and navigate available financial assistance and patient assistance programs that can help to cover co-pay and other out-of-pocket expenses
Start and stay on DOJOLVI treatment through personalized counseling and education based on the recommendations, advice, and prescription of the patient’s healthcare team
HELP YOUR PATIENTS ENROLL IN ULTRACARE
Step 1: Complete the
Start Form with your patient.
Step 2: Fax the completed*
Start Form to 1-415-723-7474.
Contact our UltraCare Guides for additional support at 1-888-756-8657.
Visit UltraCare
*Please note that a completed form is required for patient enrollment.
Download DOJOLVI materials
Resources that may provide additional insight and support for you, your patients, and their families and caregivers
Filter Resources:
All
For Healthcare Providers
For Patients and Caregivers
For Spanish-speaking Patients and Caregivers
Descargar materiales de DOJOLVI
Patient Stories
Watch stories of patients and their caregivers navigating life with LC-FAOD and DOJOLVI.
Patient Guidance for Dosing and Administering DOJOLVI

These demo videos support your patients on how to use the dosing components to prepare a DOJOLVI dose.
Watch Videos
Ver videos en Español
Patient Guidance for Proper Mixing
These videos demonstrate how to properly mix DOJOLVI into compatible semi-solid foods or liquids when preparing to take the medication orally.
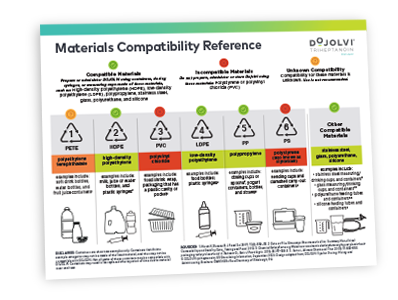
Do not administer DOJOLVI alone to avoid gastrointestinal upset. For complete information on the preparation and administration of DOJOLVI orally or enterally via a silicone or polyurethane feeding tube, please refer to Section 2.4 of the full Prescribing Information.
Watch Videos

VISIT LC-FAOD–RELATED WEBSITES
Professional organizations
Metabolic disease information and advocacy
Rare disease information and advocacy
Reference:
- Data on file. Ultragenyx Hub Data: Prescribed Patients. Ultragenyx Pharmaceutical Inc.; 2022.
Stay Informed
Sign up to receive more information and updates about DOJOLVI (triheptanoin).
FOR US HEALTHCARE PROFESSIONALS
By submitting this form, you agree to allow Ultragenyx and its agents to collect the information provided and to be contacted directly by an Ultragenyx representative. Your information will not be used for any other purpose than for a representative to respond to your information request or for us to send you other DOJOLVI updates if you have registered to receive them.
Ultragenyx will not sell, rent, or otherwise distribute your name and any personally identifiable information outside of Ultragenyx and its agents. Ultragenyx will only use your information in accordance with its Privacy Policy.
INDICATION
DOJOLVI is a medium-chain triglyceride indicated as a source of calories and fatty acids for the treatment of adult and pediatric patients with molecularly confirmed long-chain fatty acid oxidation disorders (LC-FAOD).
IMPORTANT SAFETY INFORMATION
WARNINGS AND PRECAUTIONS
Feeding Tube Dysfunction
- Feeding tube performance and functionality can degrade over time depending on usage and environmental conditions. In clinical trials, feeding tube dysfunction was reported in patients receiving triheptanoin. The contribution of DOJOLVI cannot be ruled out. Do not administer DOJOLVI in feeding tubes manufactured of polyvinyl chloride (PVC). Regularly monitor the feeding tube to ensure proper functioning and integrity.
Intestinal Malabsorption in Patients with Pancreatic Insufficiency
- Pancreatic enzymes hydrolyze triheptanoin and release heptanoate as medium-chain fatty acids in the small intestine. Low or absent pancreatic enzymes may result in reduced absorption of heptanoate subsequently leading to insufficient supplementation of medium-chain fatty acids. Avoid administration of DOJOLVI in patients with pancreatic insufficiency.
ADVERSE REACTIONS
Gastrointestinal (GI)
- The most common GI-related adverse reactions reported in the pooled safety population of Studies 1 and 2 were abdominal pain (abdominal discomfort, abdominal distension, abdominal pain, abdominal pain upper, GI pain) [60%], diarrhea [44%], vomiting [44%], and nausea [14%].
DRUG INTERACTIONS
Pancreatic Lipase Inhibitors
- Co-administration of triheptanoin with a pancreatic lipase inhibitor (e.g., orlistat) may reduce exposure to the triheptanoin metabolite, heptanoate, and reduce the clinical effect of triheptanoin. Avoid co-administration of DOJOLVI with pancreatic lipase inhibitors.
USE IN SPECIFIC POPULATIONS
Pregnancy and Lactation
- There are no available data on triheptanoin use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. There is a pregnancy safety study for DOJOLVI. If a patient becomes pregnant while receiving DOJOLVI, healthcare providers should report DOJOLVI exposure by calling Ultragenyx Pharmaceutical Inc. at 1-888-756-8657.
- There are no data on the presence of triheptanoin or its metabolites in human or animal milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the clinical need for DOJOLVI and any potential adverse effect on the breastfed infant from DOJOLVI or from the underlying maternal condition.
PATIENT COUNSELING INFORMATION
- Instruct the patient or caregiver to read the FDA-approved patient labeling, which includes information on the appropriate oral or feeding tube preparation, administration, and storage.
You may report side effects to Ultragenyx Pharmaceutical Inc. at 1-888-756-8657 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please see full Prescribing Information for a complete discussion of the risks associated with DOJOLVI.
INDICATION
DOJOLVI is a medium-chain triglyceride indicated as a source of calories and fatty acids for the treatment of adult and pediatric patients with molecularly confirmed long-chain fatty acid oxidation disorders (LC-FAOD).
IMPORTANT SAFETY INFORMATION
WARNINGS AND PRECAUTIONS
Feeding Tube Dysfunction
- Feeding tube performance and functionality can degrade over time depending on usage and environmental conditions. In clinical trials, feeding tube dysfunction was reported in patients receiving triheptanoin. The contribution of DOJOLVI cannot be ruled out. Do not administer DOJOLVI in feeding tubes manufactured of polyvinyl chloride (PVC). Regularly monitor the feeding tube to ensure proper functioning and integrity.
Intestinal Malabsorption in Patients with Pancreatic Insufficiency
- Pancreatic enzymes hydrolyze triheptanoin and release heptanoate as medium-chain fatty acids in the small intestine. Low or absent pancreatic enzymes may result in reduced absorption of heptanoate subsequently leading to insufficient supplementation of medium-chain fatty acids. Avoid administration of DOJOLVI in patients with pancreatic insufficiency.
ADVERSE REACTIONS
Gastrointestinal (GI)
- The most common GI-related adverse reactions reported in the pooled safety population of Studies 1 and 2 were abdominal pain (abdominal discomfort, abdominal distension, abdominal pain, abdominal pain upper, GI pain) [60%], diarrhea [44%], vomiting [44%], and nausea [14%].
DRUG INTERACTIONS
Pancreatic Lipase Inhibitors
- Co-administration of triheptanoin with a pancreatic lipase inhibitor (e.g., orlistat) may reduce exposure to the triheptanoin metabolite, heptanoate, and reduce the clinical effect of triheptanoin. Avoid co-administration of DOJOLVI with pancreatic lipase inhibitors.
USE IN SPECIFIC POPULATIONS
Pregnancy and Lactation
- There are no available data on triheptanoin use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. There is a pregnancy safety study for DOJOLVI. If a patient becomes pregnant while receiving DOJOLVI, healthcare providers should report DOJOLVI exposure by calling Ultragenyx Pharmaceutical Inc. at 1-888-756-8657.
- There are no data on the presence of triheptanoin or its metabolites in human or animal milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the clinical need for DOJOLVI and any potential adverse effect on the breastfed infant from DOJOLVI or from the underlying maternal condition.
PATIENT COUNSELING INFORMATION
- Instruct the patient or caregiver to read the FDA-approved patient labeling, which includes information on the appropriate oral or feeding tube preparation, administration, and storage.
You may report side effects to Ultragenyx Pharmaceutical Inc. at 1-888-756-8657 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please see full Prescribing Information for a complete discussion of the risks associated with DOJOLVI.